
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 10. g of octane is mixed with 61.9 g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 08:10
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide...
Questions

Mathematics, 06.07.2020 19:01


Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

English, 06.07.2020 19:01

Chemistry, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01


Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

Spanish, 06.07.2020 19:01

Biology, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

Mathematics, 06.07.2020 19:01

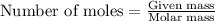
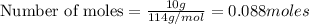
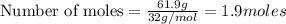
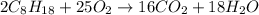
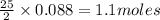 of ethane
of ethane

