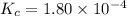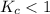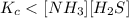Chemistry, 26.03.2020 21:33 demienarravo
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2S(g) This reaction is Reactant favored at equilibrium. Enter PRODUCT or REACTANT. The concentrations of NH3 and H2S will be at equilibrium. Enter HIGH or LOW.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2...
Questions

History, 25.11.2021 20:00

Mathematics, 25.11.2021 20:10



Mathematics, 25.11.2021 20:10

Mathematics, 25.11.2021 20:10

History, 25.11.2021 20:10



Physics, 25.11.2021 20:10




Physics, 25.11.2021 20:10

Social Studies, 25.11.2021 20:10

Mathematics, 25.11.2021 20:10


Biology, 25.11.2021 20:10


English, 25.11.2021 20:10

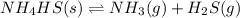
![K_c=[NH_3][H_2S]](/tpl/images/0565/8253/ff5ed.png)
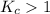 ; the reaction is product favored.
; the reaction is product favored.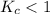 ; the reaction is reactant favored.
; the reaction is reactant favored.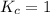 ; the reaction is in equilibrium.
; the reaction is in equilibrium.