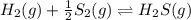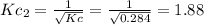Chemistry, 26.03.2020 21:03 estefaniapenalo
When heated, hydrogen sulfide gas decomposes according to the equation: 2H2S(g) → 2H2(g) + 2S2(g) A 6.75 gram sample of H2S(g) is introduced into an evacuated rigid 0.75 L container. The sealed container is heated to 283 K and 6.42 x 10 ^–2 mol of S2 gas is present at equilibrium.
a. Calculate the equilibrium concentration, in mol/L, of the H2(g) in the container at 283 K.
b. Calculate the equilibrium concentration, in mol/L, of the H2S(g) in the container at 283 K.
c. Calculate the value of the equilibrium constant, Kc, for the decomposition reaction at 283 K.
d. Calculate the partial pressure of S2(g) in atm in the container at equilibrium at 283 K.
e. Calculate the value of the equilibrium constant, Kc, for the reaction
H2(g) + 1/2 S2(g) → H2S (g) at 283 K.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
When heated, hydrogen sulfide gas decomposes according to the equation: 2H2S(g) → 2H2(g) + 2S2(g) A...
Questions



Computers and Technology, 31.01.2020 07:55

History, 31.01.2020 07:55

English, 31.01.2020 07:55

Mathematics, 31.01.2020 07:55

History, 31.01.2020 07:55


Mathematics, 31.01.2020 07:55

Mathematics, 31.01.2020 07:55


Mathematics, 31.01.2020 07:55


Computers and Technology, 31.01.2020 07:55





History, 31.01.2020 07:55

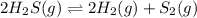
 due to stoichiometry and the reaction extent, turns out:
due to stoichiometry and the reaction extent, turns out:![K=\frac{[H_2]_{eq}^2[S_2]_{eq}}{[H_2S]_{eq}^2}](/tpl/images/0565/7677/a5ff5.png)
![[H_2S]_0=\frac{6.75g/(34g/mol)}{0.75L} =0.265M](/tpl/images/0565/7677/26ae9.png)
![[S_2]_{eq}=x=\frac{6.42x10^{-2}mol}{0.75L}=0.0856M](/tpl/images/0565/7677/c4de0.png)
![[H_2]_{eq}=2x=2*0.0856M=0.171M](/tpl/images/0565/7677/0a782.png)
![[H_2S]_{eq}=0.265M-2x=0.265M-2*0.0856M=0.0938M](/tpl/images/0565/7677/de91b.png)
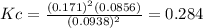
![p_{S_2}=[S_2]RT= 0.0856\frac{mol}{L}*0.082\frac{atm*L}{mol*K}*283K=1.99atm](/tpl/images/0565/7677/136fa.png)