
Chemistry, 26.03.2020 20:59 makaylashrout77
Acetylene (C2H2) gas is often used in welding torches because of the very high heat produced when it reacts with oxygen (O2) gas, producing carbon dioxide gas and water vapor. Calculate the moles of acetylene needed to produce 0.090mol of carbon dioxide. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Acetylene (C2H2) gas is often used in welding torches because of the very high heat produced when it...
Questions



History, 04.08.2019 16:00

Social Studies, 04.08.2019 16:00




Biology, 04.08.2019 16:00

Business, 04.08.2019 16:00

History, 04.08.2019 16:00


Mathematics, 04.08.2019 16:00

Chemistry, 04.08.2019 16:00

English, 04.08.2019 16:00



Social Studies, 04.08.2019 16:00


Biology, 04.08.2019 16:00

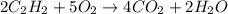
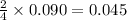 moles of acetylene
moles of acetylene

