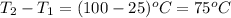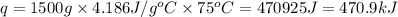How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL for the water.)
My brain wants to just start with 1.5L and use density as a conversion factor but I seriously think I'm missing something. I don't really understand the heat equation, but I'm thinking I might need to use q = mCAT?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1


Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
How much heat is required to warm 1.50L of water from 25.0C to 100.0C? (Assume a density of 1.0g/mL...
Questions







Biology, 05.10.2019 19:00

Physics, 05.10.2019 19:00




Physics, 05.10.2019 19:00




History, 05.10.2019 19:00


Mathematics, 05.10.2019 19:00

Biology, 05.10.2019 19:00

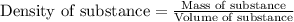
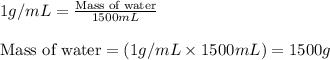
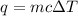
 = change in temperature =
= change in temperature =