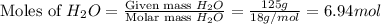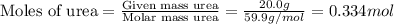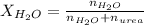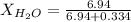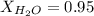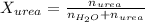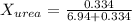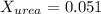Chemistry, 26.03.2020 19:59 nerikzagallegos
. You prepare a solution by adding 20.0 g Urea [(NH2)2CO] to 125 g water at 25.0oC & vapor pressure 23.76 torr. The solution vapor pressure is 22.67 torr. Calculate the molecular weight of urea. XH2O = moles H2O = Xsolute = moles urea = molar mass = calculations:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1


Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
. You prepare a solution by adding 20.0 g Urea [(NH2)2CO] to 125 g water at 25.0oC & vapor press...
Questions


Chemistry, 16.12.2020 05:10

Mathematics, 16.12.2020 05:10

English, 16.12.2020 05:10






Mathematics, 16.12.2020 05:10

Mathematics, 16.12.2020 05:10



Arts, 16.12.2020 05:10

Mathematics, 16.12.2020 05:10


Mathematics, 16.12.2020 05:10


World Languages, 16.12.2020 05:10

Spanish, 16.12.2020 05:10

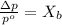
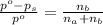
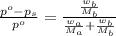
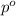 = vapor pressure of the pure solvent water = 23.76 torr
= vapor pressure of the pure solvent water = 23.76 torr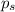 = vapor pressure of the solution = 22.67 torr
= vapor pressure of the solution = 22.67 torr = mole fraction of solute (urea)
= mole fraction of solute (urea)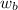 = mass of urea = 20.0 g
= mass of urea = 20.0 g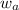 = mass of water = 125 g
= mass of water = 125 g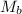 = molar mass of urea = ?
= molar mass of urea = ?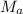 = molar mass of water = 18 g/mol
= molar mass of water = 18 g/mol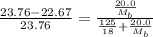
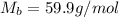
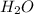 and urea.
and urea.