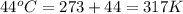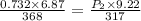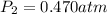Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
A 6.87-L sample of gas has a pressure of 0.732 atm and a temperature of 95 °C. The sample is allowed...
Questions


History, 24.04.2021 01:20

Mathematics, 24.04.2021 01:20


Mathematics, 24.04.2021 01:20

Mathematics, 24.04.2021 01:20


Chemistry, 24.04.2021 01:20




Mathematics, 24.04.2021 01:20

Mathematics, 24.04.2021 01:20

History, 24.04.2021 01:20

Mathematics, 24.04.2021 01:20

Health, 24.04.2021 01:20

Mathematics, 24.04.2021 01:20


Mathematics, 24.04.2021 01:20

Biology, 24.04.2021 01:20

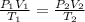
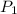 = initial pressure of gas = 0.732 atm
= initial pressure of gas = 0.732 atm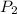 = final pressure of gas = ?
= final pressure of gas = ?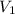 = initial volume of gas = 6.87 L
= initial volume of gas = 6.87 L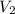 = final volume of gas = 9.22 L
= final volume of gas = 9.22 L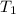 = initial temperature of gas =
= initial temperature of gas = 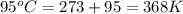
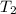 = final temperature of gas =
= final temperature of gas =