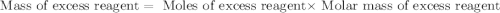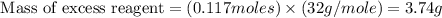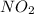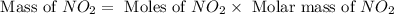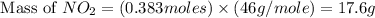Chemistry, 26.03.2020 19:41 Hilljos018
For this reaction, 11.5 g nitrogen monoxide reacts with 9.91 g oxygen gas. nitrogen monoxide (g) + oxygen (g) nitrogen dioxide (g) What is the maximum mass of nitrogen dioxide that can be formed? g What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 24.06.2019 00:00
When bonds change in chemical reactions, the characteristic properties of the substances involved
Answers: 1
You know the right answer?
For this reaction, 11.5 g nitrogen monoxide reacts with 9.91 g oxygen gas. nitrogen monoxide (g) + o...
Questions




















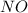
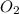 = 9.91 g
= 9.91 g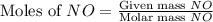
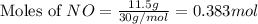
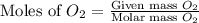
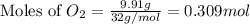
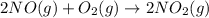
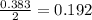 moles of
moles of