
Chemistry, 26.03.2020 18:46 briannabrewer123
When a reaction has reached equilibrium... A. Q = K B. The rate of the forward reaction and the rate of the reverse reaction are equal. C. Both the forward and reverse reactions stop occurring. D. The concentration of products and the concentration of reactants are equal. E. The forward rate constant and the reverse rate constant are equal.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
When a reaction has reached equilibrium... A. Q = K B. The rate of the forward reaction and the rate...
Questions

Mathematics, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30




Biology, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30




Law, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30

History, 10.12.2020 19:30

Health, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30

English, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30


Mathematics, 10.12.2020 19:30

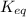 .
. cC
cC![K_{eq}=\frac{[C]^c}{[A]^a[B]^b}](/tpl/images/0565/3634/98de9.png)


