

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
How many moles of fe2o3 will be produced from 18.0 g of fe assuming o2 is available in excess...
Questions



Physics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00



English, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Biology, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00



History, 08.12.2020 01:00

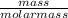
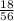 = 0.32mole
= 0.32mole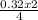 = 0.161 moles of Fe₂O₃
= 0.161 moles of Fe₂O₃

