Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
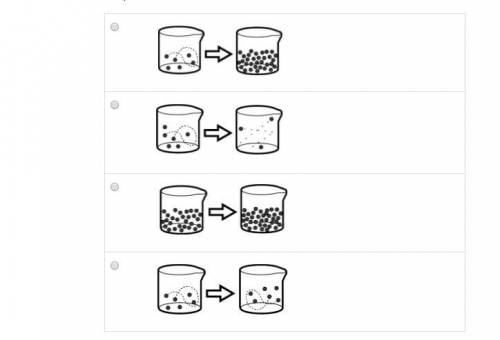
Students in a lab used a hot plate to boil water. Which of the following illustrations best represen...
Questions


Mathematics, 09.02.2020 17:51

Biology, 09.02.2020 17:51

Engineering, 09.02.2020 17:51


Chemistry, 09.02.2020 17:51


Physics, 09.02.2020 17:51

English, 09.02.2020 17:53

Mathematics, 09.02.2020 17:58



English, 09.02.2020 18:01

Chemistry, 09.02.2020 18:01


English, 09.02.2020 18:01


Mathematics, 09.02.2020 18:01


Mathematics, 09.02.2020 18:01