
Chemistry, 26.03.2020 04:28 jeffffffff
3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution's temperature increases by 19.2°C. Calculate ∆H for the dissolution of the unknown solid. (The specific heat of the solution is 4.18 J/g・°C and the density of the solution is 1.20 g/mL).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
3.15 mol of an unknown solid is placed into enough water to make 150.0 mL of solution. The solution'...
Questions

Biology, 04.01.2020 10:31

Mathematics, 04.01.2020 10:31

Mathematics, 04.01.2020 10:31




Mathematics, 04.01.2020 10:31

Mathematics, 04.01.2020 10:31


English, 04.01.2020 10:31

Mathematics, 04.01.2020 10:31

History, 04.01.2020 10:31


History, 04.01.2020 10:31

English, 04.01.2020 10:31

Mathematics, 04.01.2020 10:31




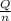 =
= 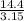 = 4.6 kj/mole
= 4.6 kj/mole

