
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a closed vessel at 400 k is charged with 1.00 atm of br2 (g), 1.00 atm of cl2 (g), and 2.00 atm of brcl (g). use q to determine which of the statements below is true.
a. the equilibrium partial pressure of br2 will be greater than 1.00 atm.
b. the reaction will go to completion since there are equal amounts of br2 and cl2.
c. the equilibrium partial pressure of brcl (g) will be greater than 2.00 atm.
d. the equilibrium partial pressures of br2, cl2, and brcl will be the same as the initial values.
e. at equilibrium, the total pressure in the vessel will be less than the initial total pressure.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a close...
Questions




Mathematics, 19.06.2020 06:57

Biology, 19.06.2020 06:57

Mathematics, 19.06.2020 06:57


History, 19.06.2020 06:57

Business, 19.06.2020 06:57


Health, 19.06.2020 06:57


Mathematics, 19.06.2020 06:57

Mathematics, 19.06.2020 06:57


Mathematics, 19.06.2020 06:57

History, 19.06.2020 06:57


Chemistry, 19.06.2020 06:57

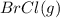 will be greater than
will be greater than 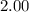 atm.
atm.![Q_{p} =\frac{[products]}{[reactants]}](/tpl/images/0564/6775/77b1b.png)
![Q_{p} = \frac{[2.00]^2}{{[1.00]}[1.00]}](/tpl/images/0564/6775/b1f67.png)
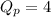
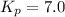
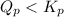 .
.

