Chemistry, 26.03.2020 02:59 lildeb8593
A cube of solid benzene (C6H6) at its melting point and weighing 10.0 g is placed in 10.0 g of water at 30 °C. Given that the heat of fusion of benzene is 9.92 kJ/mol, to what temperature will the water have cooled by the time all of the benzene has melted?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
A cube of solid benzene (C6H6) at its melting point and weighing 10.0 g is placed in 10.0 g of water...
Questions



SAT, 30.03.2020 19:33

Mathematics, 30.03.2020 19:33

Biology, 30.03.2020 19:33

Mathematics, 30.03.2020 19:33

History, 30.03.2020 19:33

Mathematics, 30.03.2020 19:33

Mathematics, 30.03.2020 19:33

Mathematics, 30.03.2020 19:33

Mathematics, 30.03.2020 19:33


Mathematics, 30.03.2020 19:33


Mathematics, 30.03.2020 19:33

Mathematics, 30.03.2020 19:33

Computers and Technology, 30.03.2020 19:33



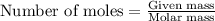
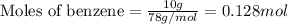
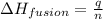
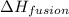 = heat of fusion of benzene = 9.92 kJ/mol = 9920 J/mol (Conversion factor: 1 kJ = 1000 J)
= heat of fusion of benzene = 9.92 kJ/mol = 9920 J/mol (Conversion factor: 1 kJ = 1000 J)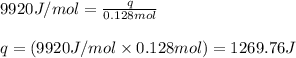
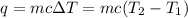
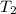 = final temperature = ?
= final temperature = ?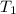 = initial temperature = 30°C
= initial temperature = 30°C