
Chemistry, 25.03.2020 23:39 sjjsksksj1590
At equilibrium, a 4.50 L container has 2.6 g of carbon, CO2 at a partial pressure of 0.0020 atm, and a total pressure of 0.572 atm. Calculate KP for this reaction at 725oC.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2


Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
At equilibrium, a 4.50 L container has 2.6 g of carbon, CO2 at a partial pressure of 0.0020 atm, and...
Questions


Social Studies, 22.04.2021 20:30




Mathematics, 22.04.2021 20:30




Mathematics, 22.04.2021 20:30

History, 22.04.2021 20:30



Social Studies, 22.04.2021 20:30



Mathematics, 22.04.2021 20:30

Mathematics, 22.04.2021 20:30


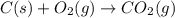
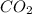 = 0.0020 atm
= 0.0020 atm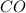 = Total pressure at equilibrium - Equilibrium pressure of
= Total pressure at equilibrium - Equilibrium pressure of 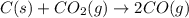
![K_p=\frac{[p_{CO}]^2}{[p_{CO_2}}](/tpl/images/0564/1099/e1319.png)
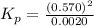
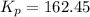
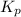 for this reaction at
for this reaction at 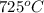 is 162.45
is 162.45

