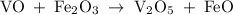Chemistry, 25.03.2020 17:43 carsondelane13
Vanadium (II) oxide with Iron (III) oxide results in Vanadium (V) oxide and Iron (II) oxide. Balance this equation

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3
You know the right answer?
Vanadium (II) oxide with Iron (III) oxide results in Vanadium (V) oxide and Iron (II) oxide. Balance...
Questions



Mathematics, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10


Chemistry, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10

English, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10

Chemistry, 08.02.2021 20:10



Computers and Technology, 08.02.2021 20:10




Mathematics, 08.02.2021 20:10