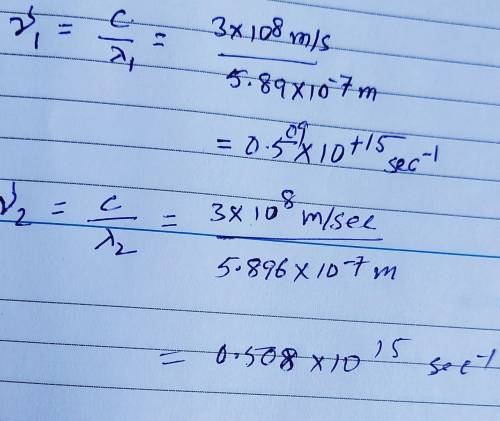Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by these lamps is actually due to two closely spaced emission lines in the visible region of the electromagnetic spectrum. One of these lines has a wavelength of 5.890 X 10⁻⁷ m, and the other line has a wavelength of 5.896 X 10⁻⁷ m. A) What are the wavelengths of these radiations in centimeters? B) Calculate the frequencies of these radiations. Show work please

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by the...
Questions


Mathematics, 16.09.2019 06:10



English, 16.09.2019 06:10

English, 16.09.2019 06:10

Health, 16.09.2019 06:10

Mathematics, 16.09.2019 06:10

Geography, 16.09.2019 06:10

History, 16.09.2019 06:10


Social Studies, 16.09.2019 06:10

History, 16.09.2019 06:10

Mathematics, 16.09.2019 06:10




Mathematics, 16.09.2019 06:10

Mathematics, 16.09.2019 06:10