
Chemistry, 25.03.2020 06:21 miacespedes
Consider the following reaction 2 N2O(g) → 2 N2(g) + O2(g) rate = k [N2O] . For an initial concentration of N2O of 0.50 M, calculate the concentration of N2O remaining after 2.0 min if k = 3.4 × 10−3 s −1 . 1. 0.17 M 2. 0.50 M 3. 0.55 M 4. 0.33 M 5. 0.66 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

You know the right answer?
Consider the following reaction 2 N2O(g) → 2 N2(g) + O2(g) rate = k [N2O] . For an initial concentra...
Questions




Mathematics, 16.12.2020 17:30

Mathematics, 16.12.2020 17:30

Mathematics, 16.12.2020 17:30

Mathematics, 16.12.2020 17:30



Mathematics, 16.12.2020 17:30

History, 16.12.2020 17:30

Mathematics, 16.12.2020 17:30


Mathematics, 16.12.2020 17:30


Physics, 16.12.2020 17:30




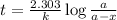
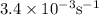
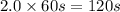
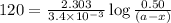
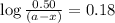
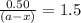

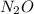 remaining after 2.0 min is 0.33 M
remaining after 2.0 min is 0.33 M

