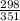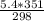4. A gas with a pressure of 5.4 atm and at 25C is raised to a new temperature of 78C.
What is...

Chemistry, 25.03.2020 06:46 2021sherodisabella
4. A gas with a pressure of 5.4 atm and at 25C is raised to a new temperature of 78C.
What is the new pressure? (Show all of your work including identifying variables,
equation and your work)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
Questions

Social Studies, 03.11.2020 14:00


Physics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00

Computers and Technology, 03.11.2020 14:00

Advanced Placement (AP), 03.11.2020 14:00




English, 03.11.2020 14:00



English, 03.11.2020 14:00



Social Studies, 03.11.2020 14:00


Social Studies, 03.11.2020 14:00


Chemistry, 03.11.2020 14:00

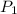 ) = 5.4 atm
) = 5.4 atm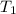 ) = 25°C = 273 + 25 = 298 K
) = 25°C = 273 + 25 = 298 K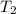 ) = 78°C = 273 + 78 = 351 K
) = 78°C = 273 + 78 = 351 K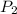 )
)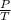 = constant
= constant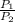 =
= 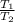
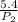 =
=