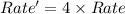Chemistry, 25.03.2020 07:01 mamas4539p79bw7
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding the concentration of M constant, the concentration of X is increased from x to 4x. Predict by what factor the rate of reaction increases.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
The reaction X + 2M → products has been found to have the rate law, rate = k[X] [M]2 . While holding...
Questions


Mathematics, 01.10.2019 09:00


Social Studies, 01.10.2019 09:00


Mathematics, 01.10.2019 09:00

Biology, 01.10.2019 09:00







Mathematics, 01.10.2019 09:00


English, 01.10.2019 09:00


Mathematics, 01.10.2019 09:00

History, 01.10.2019 09:00

Biology, 01.10.2019 09:00

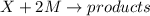
![Rate=k[X][M]^2](/tpl/images/0562/8462/02d02.png)
![Rate'=k[4X]^1[M]^2](/tpl/images/0562/8462/66381.png)
![Rate'=k[4]^1[X]^1[M]^2](/tpl/images/0562/8462/31177.png)