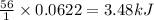Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

You know the right answer?
The enthalpy of neutralization for the reaction of a strong acid with a strong base is -56 kJ/mol of...
Questions

Mathematics, 10.04.2020 21:55


Arts, 10.04.2020 21:55



Chemistry, 10.04.2020 21:55

Mathematics, 10.04.2020 21:55








Mathematics, 10.04.2020 21:55

Mathematics, 10.04.2020 21:55




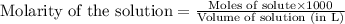 .....(1)
.....(1)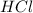 solution = 0.400 M
solution = 0.400 M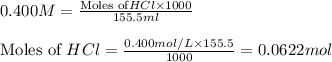
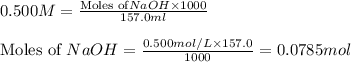

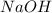
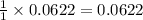 moles of
moles of