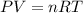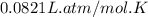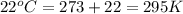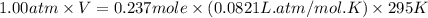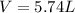Chemistry, 25.03.2020 05:36 lelseymota123
Automobile airbags contain solid sodium azide, NaN 3 , that reacts to produce nitrogen gas when heated, thus inflating the bag. 2 NaN 3 ( s ) ⟶ 2 Na ( s ) + 3 N 2 ( g ) Calculate the value of work, w , for the system if 10.3 g NaN 3 reacts completely at 1.00 atm and 22 ∘ C.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Automobile airbags contain solid sodium azide, NaN 3 , that reacts to produce nitrogen gas when heat...
Questions


History, 27.07.2021 08:50


Mathematics, 27.07.2021 08:50

Mathematics, 27.07.2021 08:50

Advanced Placement (AP), 27.07.2021 08:50

Biology, 27.07.2021 08:50



Law, 27.07.2021 08:50

Physics, 27.07.2021 08:50


Mathematics, 27.07.2021 08:50


Mathematics, 27.07.2021 08:50


Mathematics, 27.07.2021 08:50

Mathematics, 27.07.2021 08:50

Mathematics, 27.07.2021 08:50

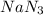
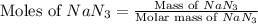
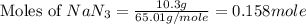
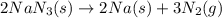
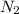
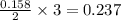 moles of
moles of