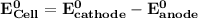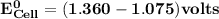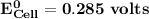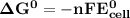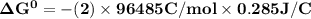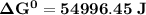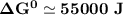Chemistry, 25.03.2020 05:26 kenzie3497
Determine ΔG° for a cell that utilizes the following reaction: Cl2(g) + 2Br–(aq) → 2Cl–(aq) + Br2(l) The standard reduction for the chlorine gas is 1.360 volts and the standard reduction for the bromine liquid is about 1.075 volts. Group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Determine ΔG° for a cell that utilizes the following reaction: Cl2(g) + 2Br–(aq) → 2Cl–(aq) + Br2(l)...
Questions

Mathematics, 17.11.2020 19:40

English, 17.11.2020 19:40

Business, 17.11.2020 19:40


English, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40

Arts, 17.11.2020 19:40

Chemistry, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40


Mathematics, 17.11.2020 19:40


Arts, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40

Health, 17.11.2020 19:40


Mathematics, 17.11.2020 19:40


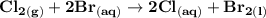
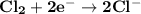
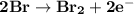
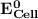 can be calculated as:
can be calculated as: