
Chemistry, 25.03.2020 05:51 kokokakahi
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30 M , and [C] = 0.500 M . The following reaction occurs and equilibrium is established: A+2B⇌C At equilibrium, [A] = 0.410 M and [C] = 0.690 M . Calculate the value of the equilibrium constant, Kc.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.600 M , [B] = 1.30...
Questions




Mathematics, 01.02.2021 20:40





Business, 01.02.2021 20:40



Mathematics, 01.02.2021 20:40

History, 01.02.2021 20:40

Mathematics, 01.02.2021 20:40


History, 01.02.2021 20:40



Mathematics, 01.02.2021 20:40

Mathematics, 01.02.2021 20:40

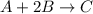
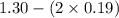
![K_{eq} = \frac{[C]}{[A][B]^{2}}](/tpl/images/0562/6350/666b5.png)
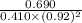
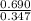
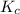 is 1.988.
is 1.988.

