
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° = -90 kJ
II) Ni(s) + 4 CO(g) = Ni(CO)4(8),
AH° = -161 kJ
will shift toward products or reactants with a
temperature increase.
1. I shifts toward reactants and II shifts
toward products.
2. Both I and II shift toward products.
3. Unable to determine
4. Both I and II shift toward reactants.
5. I shifts toward products and II shifts to-
ward reactants.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
Questions

Mathematics, 25.01.2020 05:31


Mathematics, 25.01.2020 05:31


History, 25.01.2020 05:31

Mathematics, 25.01.2020 05:31


Physics, 25.01.2020 05:31

English, 25.01.2020 05:31

History, 25.01.2020 05:31

Geography, 25.01.2020 05:31


Physics, 25.01.2020 05:31



Arts, 25.01.2020 05:31

Geography, 25.01.2020 05:31



Mathematics, 25.01.2020 05:31

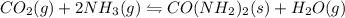 ,ΔH° = -90 kJ
,ΔH° = -90 kJ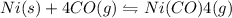 ,ΔH° = -161 kJ
,ΔH° = -161 kJ

