
The heat of solution of ammonium chloride is 15.2 kJ/mol. If a 6.134-g sample of NH4Cl is added to 65.0 mL of water in a calorimeter at 24.5°C, what is the final temperature of the solution? The specific heat of water is 4.18 J/g·°C and the heat capacity of the calorimeter is 365 J/°C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1


Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
The heat of solution of ammonium chloride is 15.2 kJ/mol. If a 6.134-g sample of NH4Cl is added to 6...
Questions


Chemistry, 22.09.2019 06:30





Mathematics, 22.09.2019 06:30

History, 22.09.2019 06:30


Biology, 22.09.2019 06:30


Physics, 22.09.2019 06:30

Mathematics, 22.09.2019 06:30



Business, 22.09.2019 06:30

Mathematics, 22.09.2019 06:30

Geography, 22.09.2019 06:30


Advanced Placement (AP), 22.09.2019 06:30


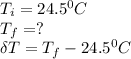
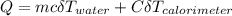
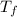 ; we have:
; we have: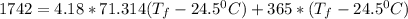
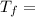 21.897 °C
21.897 °C

