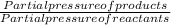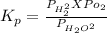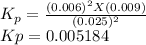The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperature until the partial pressures of H 2 O , H 2 , and O 2 reach 0.025 atm, 0.0060 atm, and 0.0090 atm, respectively at equilibrium. What is the value of the equilibrium constant at this temperature?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperat...
Questions


English, 26.12.2019 15:31


Social Studies, 26.12.2019 15:31




Business, 26.12.2019 15:31






English, 26.12.2019 15:31

Mathematics, 26.12.2019 15:31




Mathematics, 26.12.2019 15:31

Mathematics, 26.12.2019 15:31