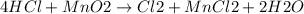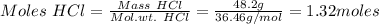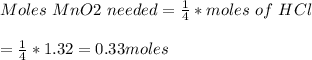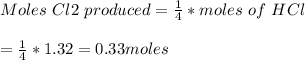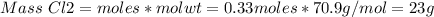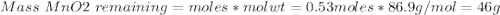Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2


Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
If 0.86 mole of mno2 and 48.2 g of hcl react, which reagent will be used up first? how many grams o...
Questions

Computers and Technology, 10.03.2020 02:13


Biology, 10.03.2020 02:13



Mathematics, 10.03.2020 02:13

Mathematics, 10.03.2020 02:13








Health, 10.03.2020 02:13



Computers and Technology, 10.03.2020 02:13

Computers and Technology, 10.03.2020 02:13