
Chemistry, 25.03.2020 01:57 wedderman6049
At a certain temperature, the reaction 2NO + Cl2 ⇌ 2NOCl has an equilibrium constant Kc of 45.0. A chemist creates a mixture with the following initial concentrations: [NO] = 0.10 M, [Cl2] = 0.20 M, and [NOCl] = 0.30 M. What will happen as the reaction begins?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
At a certain temperature, the reaction 2NO + Cl2 ⇌ 2NOCl has an equilibrium constant Kc of 45.0. A c...
Questions




Mathematics, 01.04.2021 21:40

Social Studies, 01.04.2021 21:40

Mathematics, 01.04.2021 21:40





Physics, 01.04.2021 21:40

Mathematics, 01.04.2021 21:40

Social Studies, 01.04.2021 21:40

Mathematics, 01.04.2021 21:40

Mathematics, 01.04.2021 21:40

Biology, 01.04.2021 21:40

English, 01.04.2021 21:40


Mathematics, 01.04.2021 21:40

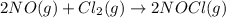 has an equilibrium constant Kc of 45.0. A chemist creates a mixture with the following initial concentrations: [NO] = 0.10 M, [Cl₂] = 0.20 M, and [NOCl] = 0.30 M. What will happen as the reaction begins?
has an equilibrium constant Kc of 45.0. A chemist creates a mixture with the following initial concentrations: [NO] = 0.10 M, [Cl₂] = 0.20 M, and [NOCl] = 0.30 M. What will happen as the reaction begins?![Q=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0562/1779/afbe9.png)
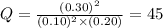
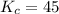
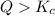 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.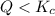 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.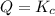 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

