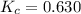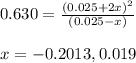Chemistry, 25.03.2020 02:08 gunnatvinson
The value of kc for the following reaction is 0.630 at 409 K N2O4(g) --> 2NO2(g) if a reaction vessel at that temperature intitially contains 0.0250 M NO2 and 0.0250 M N2O4, what is the concentration of NO2 at equilibrium

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
The value of kc for the following reaction is 0.630 at 409 K N2O4(g) --> 2NO2(g) if a reaction ve...
Questions

History, 10.03.2021 16:30







Chemistry, 10.03.2021 16:30

Mathematics, 10.03.2021 16:30


Mathematics, 10.03.2021 16:30


Geography, 10.03.2021 16:30




Mathematics, 10.03.2021 16:30

History, 10.03.2021 16:30

Chemistry, 10.03.2021 16:30

Mathematics, 10.03.2021 16:30

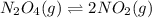
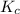 for above equation follows:
for above equation follows:![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0562/2368/271f5.png)