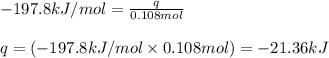Sulfur dioxide, SO 2 ( g ) , can react with oxygen to produce sulfur trioxide, SO 3 ( g ) , by the reaction 2 SO 2 ( g ) + O 2 ( g ) ⟶ 2 SO 3 ( g ) The standard enthalpies of formation for SO 2 ( g ) and SO 3 ( g ) are Δ H ∘ f [ SO 2 ( g ) ] = − 296.8 kJ / mol Δ Of [ SO3 ( g ) ] = − 395.7 kJ / mol Calculate the amount of energy in the form of heat that is produced when a volume of 2.67 L of SO 2 ( g ) is converted to 2.67 L of SO 3 ( g ) according to this process at a constant pressure and temperature of 1.00 bar and 25.0 °C . Assume ideal gas behavior.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1


Chemistry, 23.06.2019 14:00
If the molar mass of the compound is 96.69 g/mol, what is the molecular formula of the compound?
Answers: 1
You know the right answer?
Sulfur dioxide, SO 2 ( g ) , can react with oxygen to produce sulfur trioxide, SO 3 ( g ) , by the r...
Questions



Mathematics, 19.03.2021 04:30

English, 19.03.2021 04:30


Social Studies, 19.03.2021 04:30





Chemistry, 19.03.2021 04:30

Mathematics, 19.03.2021 04:30


Mathematics, 19.03.2021 04:30


Chemistry, 19.03.2021 04:30

English, 19.03.2021 04:30


Biology, 19.03.2021 04:30

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0562/0323/e893d.png)
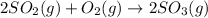
![\Delta H_{rxn}=[(2\times \Delta H_f_{(SO_3(g))})]-[(2\times \Delta H_f_{(SO_2(g))})+(1\times \Delta H_f_{(O_2(g))})]](/tpl/images/0562/0323/0e1fa.png)
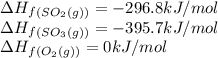
![\Delta H_{rxn}=[(2\times (-395.7))]-[(2\times (-296.8))+(1\times (0))]\\\\\Delta H_{rxn}=-197.8kJ/mol](/tpl/images/0562/0323/d0a68.png)
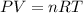
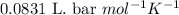
![25^oC=[25+273]K=298K](/tpl/images/0562/0323/df1f6.png)
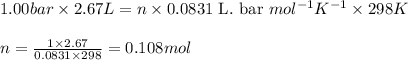
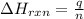
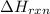 = enthalpy change of the reaction = -197.8 kJ/mol
= enthalpy change of the reaction = -197.8 kJ/mol