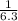Chemistry, 24.03.2020 23:12 ShelleyPSeliger
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium constant at a certain temperature is 3.10 . At this temperature, calculate the number of moles of NO 2 ( g ) that must be added to 2.30 mol SO 2 ( g ) in order to form 1.00 mol SO 3 ( g ) at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3


Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium cons...
Questions






Mathematics, 30.06.2019 20:30

Social Studies, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30


Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30


Mathematics, 30.06.2019 20:30



Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Health, 30.06.2019 20:30


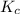 =
= ![\frac{[SO_{3} ][NO]}{[SO_{2}][NO_{2} ]}](/tpl/images/0561/8606/bc0fb.png)
![\frac{(1.00)(1.00)}{(2.30) [NO_{2} ]}](/tpl/images/0561/8606/3f85b.png) At equilibrium [SO₃] = [NO]
At equilibrium [SO₃] = [NO]