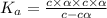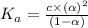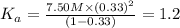Chemistry, 24.03.2020 22:32 allisonlillian
Nitric acid (HNO3) is a strong acid that is completely ionized in aqueous solutions of concentrations ranging from 1% to 10% (1.50 M ). However, in more concentrated solutions, part of the nitric acid is present as un-ionized molecules of HNO3. For example, in a 50% solution (7.50 M ) at 25°C, only 33% of the molecules of HNO3 dissociate into H+ and NO3–. What is the value of Ka for HNO3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

You know the right answer?
Nitric acid (HNO3) is a strong acid that is completely ionized in aqueous solutions of concentration...
Questions




English, 02.10.2019 03:30

English, 02.10.2019 03:30

Computers and Technology, 02.10.2019 03:30

Mathematics, 02.10.2019 03:30

History, 02.10.2019 03:30

Mathematics, 02.10.2019 03:30


English, 02.10.2019 03:30


Social Studies, 02.10.2019 03:30


Mathematics, 02.10.2019 03:30

Mathematics, 02.10.2019 03:30

English, 02.10.2019 03:40



Geography, 02.10.2019 03:40

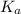 of the nitric acid is 1.2.
of the nitric acid is 1.2.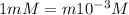
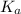
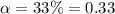
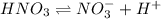
![K_a=\frac{[NO_3^{-}][H^+]}{[HNO_3]}](/tpl/images/0561/7216/4e976.png)