
You're provided with a bottle labled [CoCl2.6H2O] = 0.054 M in 4.60 M HCl. You heat a small volume of the solution in a hot water bath to 50 ∘C. If you determine that [CoCl42-] = 0.034 M at 50 ∘C at equilibrium, what must be the equilibrium concentration of [Cl-] at 50 ∘C?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
You're provided with a bottle labled [CoCl2.6H2O] = 0.054 M in 4.60 M HCl. You heat a small volume o...
Questions







Mathematics, 10.12.2020 01:00


Spanish, 10.12.2020 01:00


History, 10.12.2020 01:00




Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Engineering, 10.12.2020 01:00



History, 10.12.2020 01:00

![[CoCl_2.6H_2O]](/tpl/images/0561/6622/227f6.png) = 0.054 M
= 0.054 M![[CoCl_4]^{2-}](/tpl/images/0561/6622/84955.png) = 0.034 M
= 0.034 M![[CoCl_2.6H_2O]+2HCl\rightarrow H_2[CoCl_4]+6H_2O](/tpl/images/0561/6622/defc4.png)
![[CoCl_2.6H_2O]+2Cl^-\rightarrow [CoCl_4]^{2-}+6H_2O](/tpl/images/0561/6622/500e9.png)
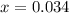
 = (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M
= (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M

