
Chemistry, 24.03.2020 21:37 shakkahdbwjsjs3068
In the making stock solutions from solids problem, make a 100 ml solution that is 2.5 M of the Cl- ion using NaCl and make a 100 mL solution that is 0.5 M of the Cl- ion using MgCl2. Show your calculations and give details about the equipment used, which solutions you mixed together, and amounts of each.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
In the making stock solutions from solids problem, make a 100 ml solution that is 2.5 M of the Cl- i...
Questions

Computers and Technology, 12.07.2019 09:30

Biology, 12.07.2019 09:30


Mathematics, 12.07.2019 09:30



Mathematics, 12.07.2019 09:30

Social Studies, 12.07.2019 09:30


Physics, 12.07.2019 09:30

Mathematics, 12.07.2019 09:30

Mathematics, 12.07.2019 09:30

Chemistry, 12.07.2019 09:30


History, 12.07.2019 09:30

History, 12.07.2019 09:30



Mathematics, 12.07.2019 09:30

Physics, 12.07.2019 09:30

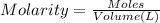
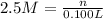
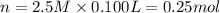
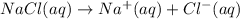
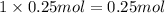 of NaCl
of NaCl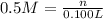
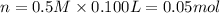
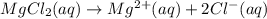
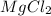 ,then 0.05 mol of chloride ions will be obtained from :
,then 0.05 mol of chloride ions will be obtained from :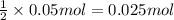 of
of 

