

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1

You know the right answer?
At a certain temperature, the equilibrium constant, K c , is 0.00376 for the reaction Cl 2 ( g ) − ⇀...
Questions

Biology, 06.02.2021 01:50

Mathematics, 06.02.2021 01:50

Mathematics, 06.02.2021 01:50


Mathematics, 06.02.2021 01:50

Mathematics, 06.02.2021 01:50

Health, 06.02.2021 01:50



Mathematics, 06.02.2021 01:50







Arts, 06.02.2021 01:50

Biology, 06.02.2021 01:50

Chemistry, 06.02.2021 01:50

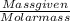
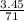
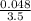 M = 0.013 molar
M = 0.013 molar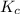 =
= ![\frac{[Cl]^{2} }{[Cl_{2} ]}](/tpl/images/0561/4874/ed9f2.png)


