Chemistry, 24.03.2020 21:04 rosieposie27
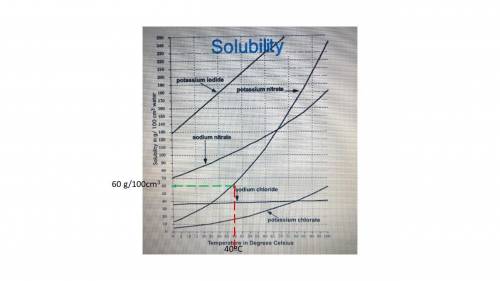
The graph shows the solubility of several different salts in water, across a range of temperatures. According to the
graph, which salt can dissolve at a concentration of about 60 g/100 cm3 of water at 40 degrees Celsius?
A) sodium nitrate
B) sodium chloride
C) potassium iodide
D) potassium nitrate


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The graph shows the solubility of several different salts in water, across a range of temperatures....
Questions

History, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Chemistry, 12.12.2020 16:40


History, 12.12.2020 16:40


History, 12.12.2020 16:40

Chemistry, 12.12.2020 16:40


Chemistry, 12.12.2020 16:40

English, 12.12.2020 16:40







Physics, 12.12.2020 16:40