
Chemistry, 24.03.2020 20:39 decoreyjpaipxv
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl ( aq ) HCl(aq) , as described by the chemical equation MnO 2 ( s ) + 4 HCl ( aq ) ⟶ MnCl 2 ( aq ) + 2 H 2 O ( l ) + Cl 2 ( g ) MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+ Cl2(g) How much MnO 2 ( s ) MnO2(s) should be added to excess HCl ( aq ) HCl(aq) to obtain 385 mL Cl 2 ( g ) 385 mL Cl2(g) at 25 °C and 765 Torr 765 Torr ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions

English, 18.03.2020 22:10



Mathematics, 18.03.2020 22:10







English, 18.03.2020 22:10

Mathematics, 18.03.2020 22:10



English, 18.03.2020 22:10

Mathematics, 18.03.2020 22:11

English, 18.03.2020 22:11



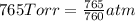
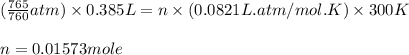
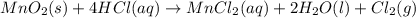
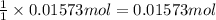 of manganese dioxide
of manganese dioxide

