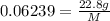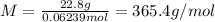A 22.8-gram sample of gas is found to have a volume of 1.5 liters at 287 K and 0.98
atm. What...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Questions

Arts, 24.07.2019 18:30





Mathematics, 24.07.2019 18:30

History, 24.07.2019 18:30

History, 24.07.2019 18:30





History, 24.07.2019 18:30





History, 24.07.2019 18:30

Chemistry, 24.07.2019 18:30