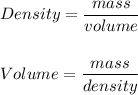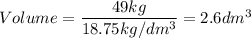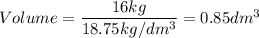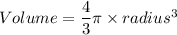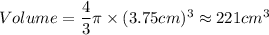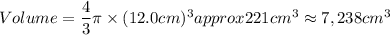Chemistry, 24.03.2020 19:58 AgarioEdit
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below, providing the explanation to your answers. a. What volume is occupied by a critical mass of 49 kg of this alloy? b. The critical mass can be decreased to 16 kg if the alloy is surrounded by a layer of natural uranium (which acts as a neutron reflector). What is the volume of such smaller mass? Compare your answers to the approximate volumes of a baseball, a volleyball, and a basketball.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below...
Questions


Physics, 08.04.2020 21:23


Mathematics, 08.04.2020 21:23



Social Studies, 08.04.2020 21:23

History, 08.04.2020 21:23



Mathematics, 08.04.2020 21:23

Chemistry, 08.04.2020 21:23






History, 08.04.2020 21:23