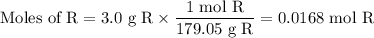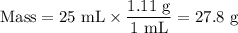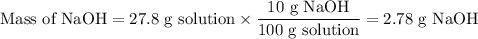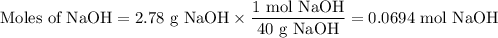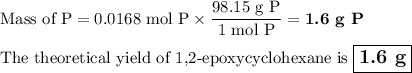Chemistry, 24.03.2020 20:15 soccerhannah290
The theoretical yield of 1,2-epoxycyclohexane is grams, when starting with 3.0 grams of trans-2-bromocyclohexanol. (Enter the number using 3 significant figures, i. e. 1.22) Given: 3.0 g of trans-2-bromocyclohexanol FW: 179.05 25 mL of 10% NaOH FW: 40 and density: 1.11 g/mL 1,2-epoxycyclohexane FW: 98.15

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1
You know the right answer?
The theoretical yield of 1,2-epoxycyclohexane is grams, when starting with 3.0 grams of trans-2-bro...
Questions

Mathematics, 22.02.2021 20:40


Mathematics, 22.02.2021 20:40


Spanish, 22.02.2021 20:40

Spanish, 22.02.2021 20:40

Computers and Technology, 22.02.2021 20:40




Mathematics, 22.02.2021 20:40

Mathematics, 22.02.2021 20:40


Mathematics, 22.02.2021 20:40

Mathematics, 22.02.2021 20:40

Mathematics, 22.02.2021 20:40



Arts, 22.02.2021 20:40

Health, 22.02.2021 20:40