

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
2 H2(g) + S2(g) equilibrium reaction arrow 2 H2S(g) At a certain temperature, Kc = 1.30 ✕ 1010 for t...
Questions

Geography, 29.11.2019 14:31

English, 29.11.2019 14:31


Chemistry, 29.11.2019 14:31


Mathematics, 29.11.2019 14:31

Business, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31

Social Studies, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31

History, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31


Biology, 29.11.2019 14:31

Mathematics, 29.11.2019 14:31


Social Studies, 29.11.2019 14:31

Social Studies, 29.11.2019 14:31

History, 29.11.2019 14:31

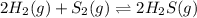
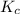 for above equation follows:
for above equation follows:![K_{c}=\frac{[H_2S]^2}{[H_2]^2[S_2]}](/tpl/images/0561/3034/d1522.png)
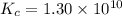
![[H_2]_{eq}=0.00400M](/tpl/images/0561/3034/ad07b.png)
![[S_2]_{eq}=0.00270M](/tpl/images/0561/3034/9642e.png)
![1.30\times 10^{10}=\frac{[H_2S]^2}{(0.00400)^2\times 0.00270}](/tpl/images/0561/3034/e2a23.png)
![[H_2S]_{eq}=23.7,-23.7](/tpl/images/0561/3034/68e27.png)


