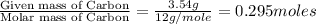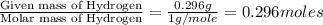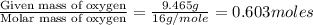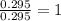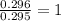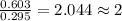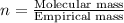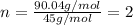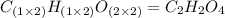Chemistry, 24.03.2020 19:38 cakeisalie6865
A 13.30 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 13.00 grams of CO2 and 2.662 grams of H2O are produced. In a separate experiment, the molar mass is found to be 90.04 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1


Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
A 13.30 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis...
Questions


Mathematics, 05.02.2021 23:30


Mathematics, 05.02.2021 23:30



Mathematics, 05.02.2021 23:30

Computers and Technology, 05.02.2021 23:30


Mathematics, 05.02.2021 23:30


Chemistry, 05.02.2021 23:30

History, 05.02.2021 23:30


Arts, 05.02.2021 23:30

Mathematics, 05.02.2021 23:30


Mathematics, 05.02.2021 23:30


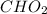 and
and 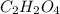
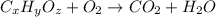
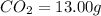
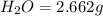
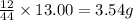 of carbon will be contained.
of carbon will be contained.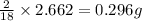 of hydrogen will be contained.
of hydrogen will be contained.