Chemistry, 24.03.2020 19:14 roseemariehunter12
Initially a NaOH solution was standardized by titration with a sample of potassium hydrogenphthalate, KHC8H4O4, a monoprotic acid often used as a primary standard. A sample of pureKHC8H4O4 weighing 1.518 grams was dissolved in water and titrated with the NaOH solution. Toreach the equivalence point, 26.90 milliliters of base was required. Calculate the molarity of theNaOH solution. (Molecular weight: KHC8H4O4 = 204.2)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1


Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Initially a NaOH solution was standardized by titration with a sample of potassium hydrogenphthalate...
Questions




Mathematics, 26.06.2020 16:01




Computers and Technology, 26.06.2020 16:01

World Languages, 26.06.2020 16:01








Mathematics, 26.06.2020 16:01



English, 26.06.2020 16:01

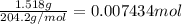
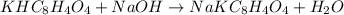
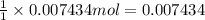 of NaOH
of NaOH