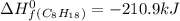For a particular isomer of C8H18, the combustion reaction produces 5113.3 kJ of heat per mole of C8H18(g) consumed, under standard conditions. C8H18(g)+252O2(g)⟶8CO2(g)+9H2O(g)ΔH ∘rxn=−5113.3 kJ/mol What is the standard enthalpy of formation of this isomer of C8H18(g)? ΔH∘f=

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
For a particular isomer of C8H18, the combustion reaction produces 5113.3 kJ of heat per mole of C8H...
Questions

Geography, 24.01.2022 19:50


Computers and Technology, 24.01.2022 19:50


Mathematics, 24.01.2022 19:50

Mathematics, 24.01.2022 19:50


French, 24.01.2022 19:50

English, 24.01.2022 19:50


Mathematics, 24.01.2022 19:50

Mathematics, 24.01.2022 19:50


Mathematics, 24.01.2022 19:50

Mathematics, 24.01.2022 19:50




Biology, 24.01.2022 19:50

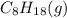 is -210.9 kJ
is -210.9 kJ
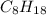 .
.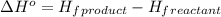
![\Delta H^o=[n_{CO_2}\times \Delta H_f^0_{(CO_2)}+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]-[n_{O_2}\times \Delta H_f^0_{(O_2)+n_{C_8H_{18}}\times \Delta H_f^0_{(C_8H_{18})}]](/tpl/images/0561/1035/28ca9.png)
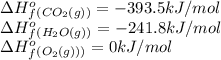
![-511.3kJ/mol=[(8\times -393.5)+(9\times -241.8)]-[(\frac{25}{2}\times 0)+(1\times \Delta H_f^0_{(C_8H_{18})}](/tpl/images/0561/1035/6b6c5.png)