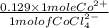Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
A bottle in lab is labeled [CoCl2.6H2O] = 0.652 M in 8.433 M HCl. If you determine [CoCl42-] to be 0...
Questions

Mathematics, 05.05.2021 07:40

English, 05.05.2021 07:40

Mathematics, 05.05.2021 07:40



Mathematics, 05.05.2021 07:40




Arts, 05.05.2021 07:40

Mathematics, 05.05.2021 07:40









![[Co(H_{2}O)_{6}]^{2} + 4Cl^{-} \rightleftharpoons [CoCl_{4}]^{2-} + 6H_{2}O](/tpl/images/0561/0378/1b7cc.png)
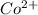 as follows.
as follows.![\frac{0.652 \times 1 mole Co^{2+}}{1 mole [CoCl(H_{2}O)_{6}]^{2+}}](/tpl/images/0561/0378/d9b5b.png)