
The reaction between carbon tetrachloride, CCl4, and water, H2O, to form carbon dioxide, CO2, and hydrogen chloride, HCl, has a ΔrG∘ value of −232 kJ mol−1, and so is thermodynamically favoured. But when you mix carbon tetrachloride with water, no change is observed. What is a possible explanation for this?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
The reaction between carbon tetrachloride, CCl4, and water, H2O, to form carbon dioxide, CO2, and hy...
Questions


History, 24.05.2021 01:00


Mathematics, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00


Mathematics, 24.05.2021 01:00



Mathematics, 24.05.2021 01:00

English, 24.05.2021 01:00

World Languages, 24.05.2021 01:00

Physics, 24.05.2021 01:00

History, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00




Engineering, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00

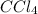 and
and 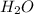 is thermodynamically favored due to negative enthalpy change but the high activation energy does not allow the reaction to take place by simple mixing.
is thermodynamically favored due to negative enthalpy change but the high activation energy does not allow the reaction to take place by simple mixing.

