Chemistry, 24.03.2020 17:44 hughesbella
Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 7.9 × 10-5, Ka2 = 1.6 × 10-12). Calculate the pH of a solution that contains 2.1 mg acid per mL water. (Assume that only the first ionization is important in determining pH.)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1
You know the right answer?
Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 7.9 × 10-5, Ka2 = 1.6 × 10-12). Calculat...
Questions



History, 16.07.2019 16:30


World Languages, 16.07.2019 16:30



Biology, 16.07.2019 16:30


Business, 16.07.2019 16:30


History, 16.07.2019 16:30


History, 16.07.2019 16:30


History, 16.07.2019 16:30

Social Studies, 16.07.2019 16:30


History, 16.07.2019 16:30

Mathematics, 16.07.2019 16:30

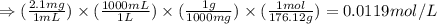
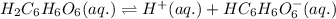
![Ka_1=\frac{[H^+][HC_6H_6O_6^{-}]}{[H_2C_6H_6O_6]}](/tpl/images/0560/9865/81fa9.png)
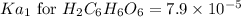
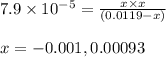
![pH=-\log[H^+]](/tpl/images/0560/9865/cf945.png)
![[H^+]=0.00093M](/tpl/images/0560/9865/52e6b.png)