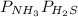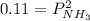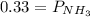Chemistry, 24.03.2020 16:55 ynclankaedon
Ammonium hydrogen sulfide NH4HS(s) decomposes on heating according to the reaction NH4HS(s) ↔ NH3(g) + H2S(g) At 25 °C the equilibrium cnstant of the reaction is Kp = 0.11. What is the total pressure at that temperature in a flask that was initially partially filled with a sizeable amount of NH4HS(s)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Ammonium hydrogen sulfide NH4HS(s) decomposes on heating according to the reaction NH4HS(s) ↔ NH3(g)...
Questions



Mathematics, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40

History, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40



Mathematics, 05.02.2021 20:40

Mathematics, 05.02.2021 20:40

Mathematics, 05.02.2021 20:40

Engineering, 05.02.2021 20:40


Spanish, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40