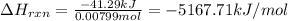Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8)(C10H8). When 1.025 gg of naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘C∘C to 32.33 ∘C Find ΔErxn for the combustion of naphthalene. The heat capacity of the calorimeter, determined in separate experiment, is 5.11kJ/∘C .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8)(C10H8). When 1.025 gg of nap...
Questions

Mathematics, 28.08.2019 06:30

Business, 28.08.2019 06:30



Computers and Technology, 28.08.2019 06:30



Social Studies, 28.08.2019 06:30

Social Studies, 28.08.2019 06:30





Chemistry, 28.08.2019 06:30

Biology, 28.08.2019 06:30

History, 28.08.2019 06:30



Geography, 28.08.2019 06:30

Health, 28.08.2019 06:30

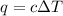
 = change in temperature =
= change in temperature = 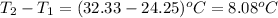
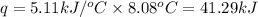
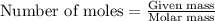
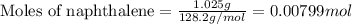
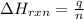
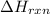 = enthalpy change of the reaction
= enthalpy change of the reaction